Lithium-IRON. The New Dominant Lithium Chemistry of the 21st Century.
Why Lithium-IRON is Taking Over -VS- ION Batteries
- A lithium-ion (Li-ion) battery is a type of rechargeable battery that relies on the movement of lithium ions between the positive and negative electrodes to generate electrical current. Li-ion batteries are widely used in various electronic devices, from smartphones and laptops to electric vehicles and energy storage systems. They are popular due to their high energy density, low self-discharge rate, and rechargeable nature.
- Anode – this is the negative electrode usually made of carbon graph-ite material. During discharge (usage) lithium ION’s move from the anode to the cathode (positive electrode) releasing electrical energy.
- Cathode – this is the positive electrode made of metal oxide such as lithium cobalt oxide (LiCoO2), lithium manganese oxide. During dis-charge the lithium ions move from the anode to the cathode releas-ing electrical energy.
- Separator – permeable membrane that separates the anode and cathode while allowing the passage of lithium ions. Prevents direct contact of the electrodes that could cause a short circuit.
- Electrolyte – Non-Aqueous solution of lithium salt dissolved in a sol-vent that helps the movement of lithium ions from the positive to the negative electrodes.
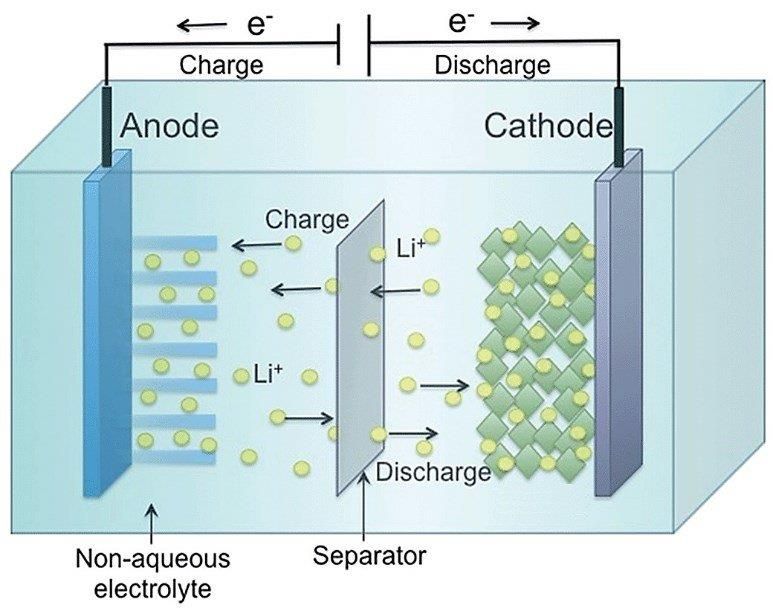
Here is a picture to help with the long description outline above. Relatively simple system that operates in a similar way to lead acid.
- Discharge – when the battery is being used for operation of a piece of equipment as described above. This movement of IONS generates the electrical current used by the equipment.
- Charging – the reverse effect of discharge where the IONS move in the opposite direction when connected to a charger.

- Sealed System – batteries are sealed shut – there are no external cells or systems other than the connector and some external sensors that are exposed.
- Maintenance free – for the most part, these batteries do not need anything in the way of maintenance.
- No water needed.
- No equalization of the battery.
- No resistance to charge (battery memory in lead acid)
- Long cycle life – unlike lead acid batteries, lithium batteries can be “topped off” with a short charge to keep the battery in a constant state of charge with no negative effects on the battery itself.
- Opportunity charging – designed for this method vs traditional cycling like lead acid.
- Fast charging – 2.5 times as fast as a conventional lead acid battery.
- High energy density – lithium-ION batteries pack a lot into a little package making them ideal for phones, laptops, and other types of products. Small battery with lots of power.
- Made them ideal for initial use in about everything.
- Rechargeable nature – Designed for opportunity charging!
- Breaks, lunch, shift changes, bathroom, etc. the more you plug them in = the more runtime you have!
- Nontraditional “cycle life” vs a lead acid battery
- Small devices are high demand and high drain on the battery causing excessive heating of the battery itself. Older first-generation Lithium batteries caught fire, or the packs expanded and burst open due to the heat developed by the devices they were installed in.
- Industrial equipment is no exception here as hydraulic demand and constant loading of the battery will generate heat. They may not catch fire, but the heat will erode the battery.

So why would a lesser powered system in IRON Phosphate be used?
Some reasons why:
- Safety – if there is too much heat developed in Lithium ION = Unstoppable Exothermic Chain Reaction resulting in FIRE. This can also happen if the battery is punctured or damaged.
- From 140-284F the battery could release all its en-ergy at once! This is what happened see pic:
- Lithium ION is hazardous to skin, eyes etc. deadly if swallowed.
- Specialty recycling requirements
- Precious metals like cobalt and nickel used here are hazardous, can be toxic to human health. Limited re-sources will run out.
Remember this from 2016 Samsung Galaxy phones?

This is the same technology in an industrial lithium-ION battery, but now it's also pushing the limits of the battery capacity for power and fastest charge.
Lithium IRON Phosphate does not have this WEAKNESS!
- LFP has excellent thermal and chemical stability!
- Will not combust when short circuited or damaged!
- Nontoxic materials are used in construction = safer.
- Longer cycle life & longer shelf life when not in use.
- Stays cooler when being used and charged.
- Less costly vs Lithium ION.
This is what you will see with NOBLELIFT lithium IRON batteries. The blue box is the battery sealed shut with minimal connections. There are some key features to this battery:
Key Features of the NOBLELIFT lithium IRON Phosphate Blue Battery:
- Data Plate - all information regarding the voltage, specifications, amperage etc. of the battery (Yellow Arrow below)
- QR Code - located below the data plate, can be scanned to show the current state of charge of the battery as it sits. (Red Arrow below)
- App is called "Battery Pack Assistant"
- iPhone, Android Compatible
- QR Code Icon

- Battery Alarm - this is the audible alarm that would alert an operator to put the battery on charge. (Yellow arrow below)
- Activated at a 10% S.O.C (State of Charge) this is the last effort to get the battery plugged in.
- Lift lockout will occur before 10% power as a safety precaution.
- Safety information – (Light blue arrow below)
- Antenna – Communicates with Bluetooth for App (Cream arrow below)
- Handles – help guide in the event of removal of the battery (Orange arrow below)
- Maintenance Free and designed to be OPPORTUNITY CHARGED
- 10 Year or 20,000 hour battery cell warranty on all 48 and 80V Lithium-IRON Phosphate batteries








Share On: